Research Highlights
Sphingosylphosphorylcholine induces itch via activation of TRPM3 and TRPA1 in mice
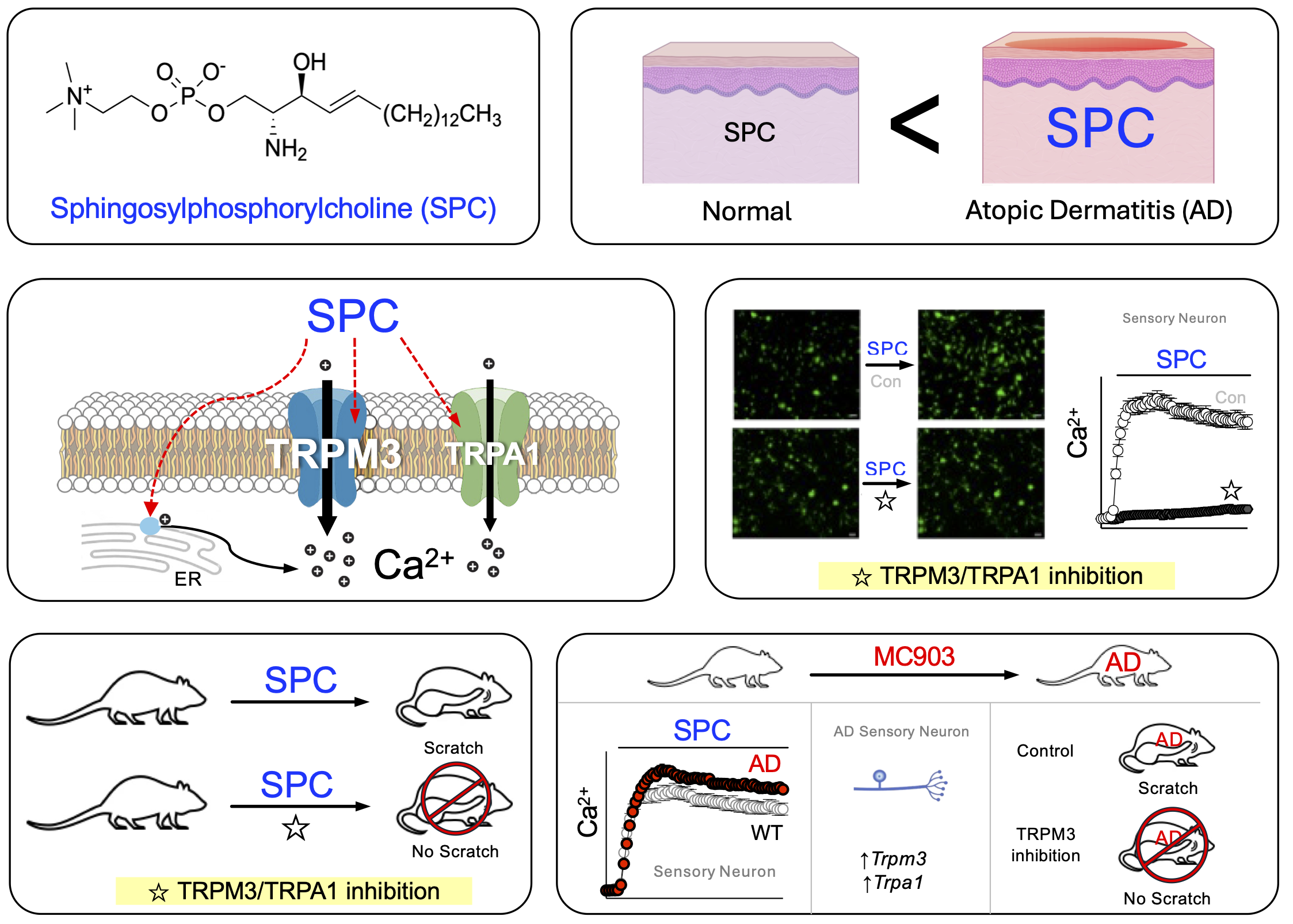
Itch is a prevalent symptom in atopic dermatitis (AD), often leading to a strong urge to scratch. Elevated levels of sphingosylphosphorylcholine (SPC) are found in the stratum corneum of AD patients, and while SPC is known to induce itch, its molecular targets are not well understood. This study aims to identify the signaling pathway of SPC-induced itch under AD conditions. We demonstrate that SPC specifically activates the Transient Receptor Potential Melastatin 3 (TRPM3) channel in sensory neurons. In HEK293T cells expressing TRPM3, SPC treatment caused a significant increase in intracellular calcium, which was inhibited by TRPM3 antagonists. Among various TRP channels tested, TRPM3 exhibited the highest reactivity to SPC, followed by TRPA1. Molecular docking analysis also supported interactions between SPC and both TRPM3 and TRPA1. In an AD mouse model, SPC-induced responses were dependent on TRPM3 and TRPA1, and the expression of these channels increased in dorsal root ganglion neurons. SPC-induced scratching behaviors were significantly reduced by TRPM3 and TRPA1 antagonists, with TRPM3 playing a critical role in spontaneous scratching. This study identifies TRPM3 and TRPA1 as key mediators of SPC-induced itch, providing potential therapeutic targets for treating itch in AD patients.
Song DE, Rawal D, Lee WJ, Shim WS. Sphingosylphosphorylcholine induces itch via activation of TRPM3 and TRPA1 in mice.
Biochem Pharmacol. 2025 Jul;237:116952.
https://doi.org/10.1016/j.bcp.2025.116952
Antipruritic effect of ursolic acid through MRGPRX2/MrgprB2-dependent inhibition of mast cell degranulation and reduced TSLP production
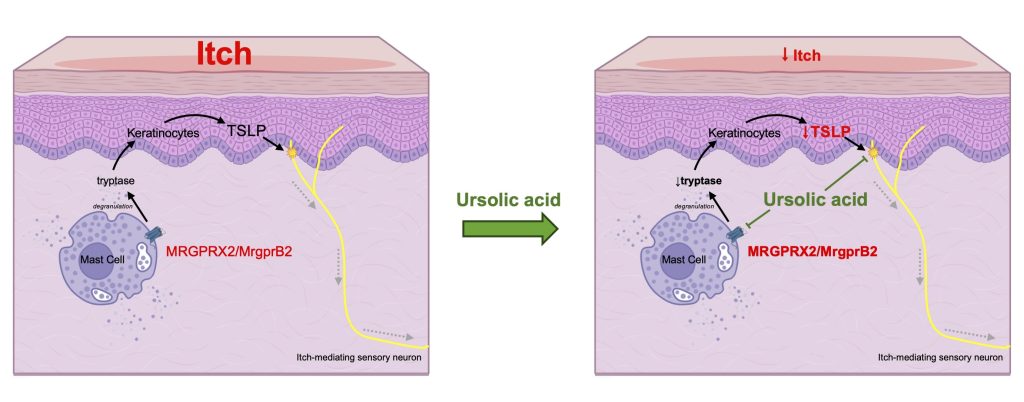
Ursolic acid (UA), a pentacyclic triterpene, exhibits diverse pharmacological effects, including potential treatment for allergic diseases. It downregulates thymic stromal lymphopoietin (TSLP) and disrupts mast cell signaling pathways. However, the exact molecular mechanism by which UA interferes with mast cell action remains unclear. Therefore, the current study aimed to uncover molecular entities underlying the effect of UA on mast cells and its potential antipruritic effect, specifically investigating its modulation of key molecules such as TRPV4, PAR2, and MRGPRX2, which are involved in TSLP regulation and sensation. Calcium imaging experiments revealed that UA pretreatment significantly suppressed MRGPRX2 activation (and its mouse orthologue MrgprB2), a G protein-coupled receptor predominantly expressed in mast cells. Molecular docking predictions suggested potential interactions between UA and MRGPRX2/MrgprB2. UA pretreatment also reduced mast cell degranulation through MRGPRX2 and MrgprB2-dependent mechanisms. In a dry skin mouse model, UA administration decreased tryptase and TSLP production in the skin, and diminished TSLP response in the sensory neurons. While PAR2 and TRPV4 activation enhances TSLP production, UA did not inhibit their activity. Notably, UA attenuated compound 48/80-induced scratching behaviors in mice and suppressed spontaneous scratching in a dry skin model. The present study confirms the effective inhibition of UA on MRGPRX2/MrgprB2, leading to reduced mast cell degranulation and suppressed scratching behaviors. These findings highlight the potential of UA as an antipruritic agent for managing various allergy- or itch-related conditions.
Cha J, Ryu J, Rawal D, Lee WJ, Shim WS. Antipruritic effect of ursolic acid through MRGPRX2/MrgprB2-dependent inhibition of mast cell degranulation and reduced TSLP production.
Eur J Pharmacol. 2024 Oct 15;981:176896
https://doi.org/10.1016/j.ejphar.2024.176896.
FSLLRY-NH2, a protease-activated receptor 2 (PAR2) antagonist, activates mas-related G protein-coupled receptor C11 (MrgprC11) to induce scratching behaviors in mice
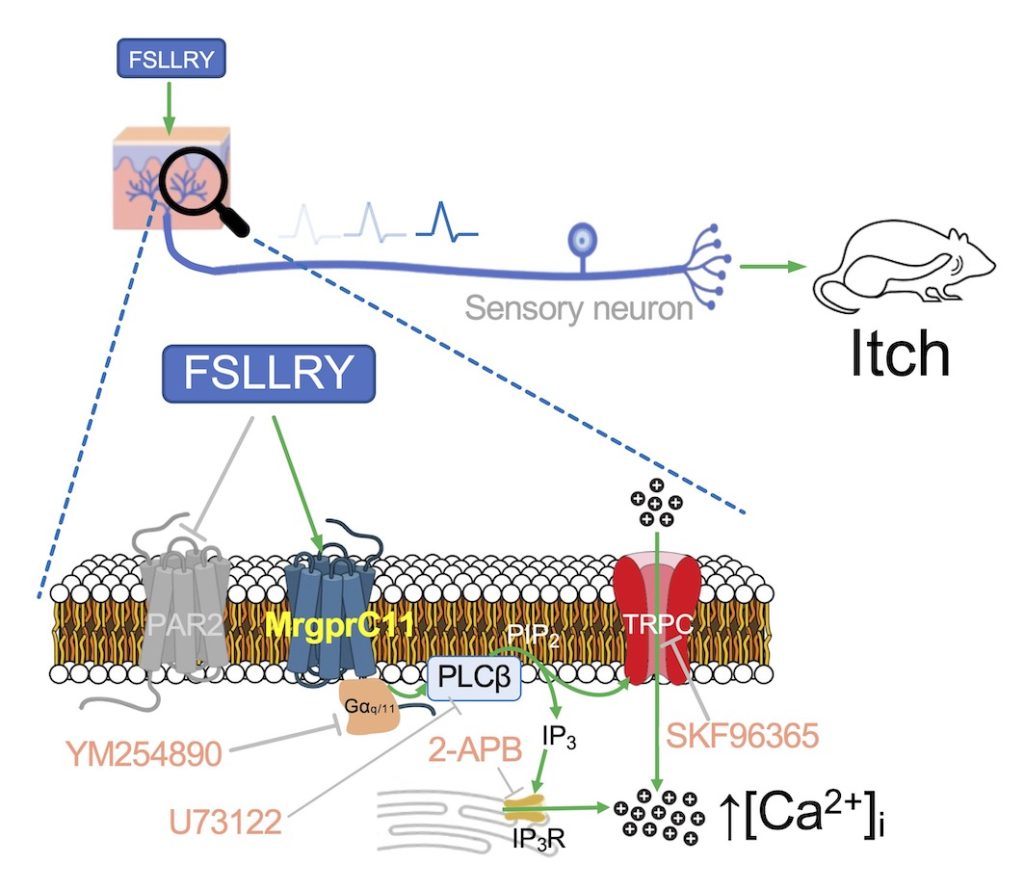
Protease-activated receptor 2 (PAR2), a type of G protein-coupled receptor (GPCR), plays a significant role in pathophysiological conditions such as inflammation. A synthetic peptide SLIGRL-NH2 (SLIGRL) can activate PAR2, while FSLLRY-NH2 (FSLLRY) is an antagonist. A previous study showed that SLIGRL activates both PAR2 and mas-related G protein-coupled receptor C11 (MrgprC11), a different type of GPCR expressed in sensory neurons. However, the impact of FSLLRY on MrgprC11 and its human ortholog MRGPRX1 was not verified. Hence, the present study aims to verify the effect of FSLLRY on MrgprC11 and MRGPRX1. It was surprisingly discovered that FSLLRY specifically activates MrgprC11 in a dose-dependent manner, but not other MRGPR subtypes. Furthermore, FSLLRY also moderately activated MRGPRX1. FSLLRY stimulates downstream pathways including Gαq/11, phospholipase C, IP3 receptor, and TRPC ion channels to evoke an increase in the intracellular calcium levels. The molecular docking analysis predicted that FSLLRY interacts with the orthosteric binding pocket of MrgprC11 and MRGPRX1. Finally, FSLLRY activated primary cultures of mouse sensory neurons, and induced scratching behaviors in mice. The present study has revealed that FSLLRY is capable of triggering itch sensation through activation of MrgprC11. This finding highlights the importance of considering the unexpected activation of MRGPRs in future therapeutic approaches aimed at the inhibition of PAR2.
In Kim H, Lee GB, Song DE, Sanjel B, Lee WJ, Shim WS. FSLLRY-NH2, a protease-activated receptor 2 (PAR2) antagonist, activates mas-related G protein-coupled receptor C11 (MrgprC11) to induce scratching behaviors in mice.
Life Sci. 2023 Jul 15;325:121786.
Glucosylsphingosine evokes pruritus via activation of 5-HT2A receptor and TRPV4 in sensory neurons.
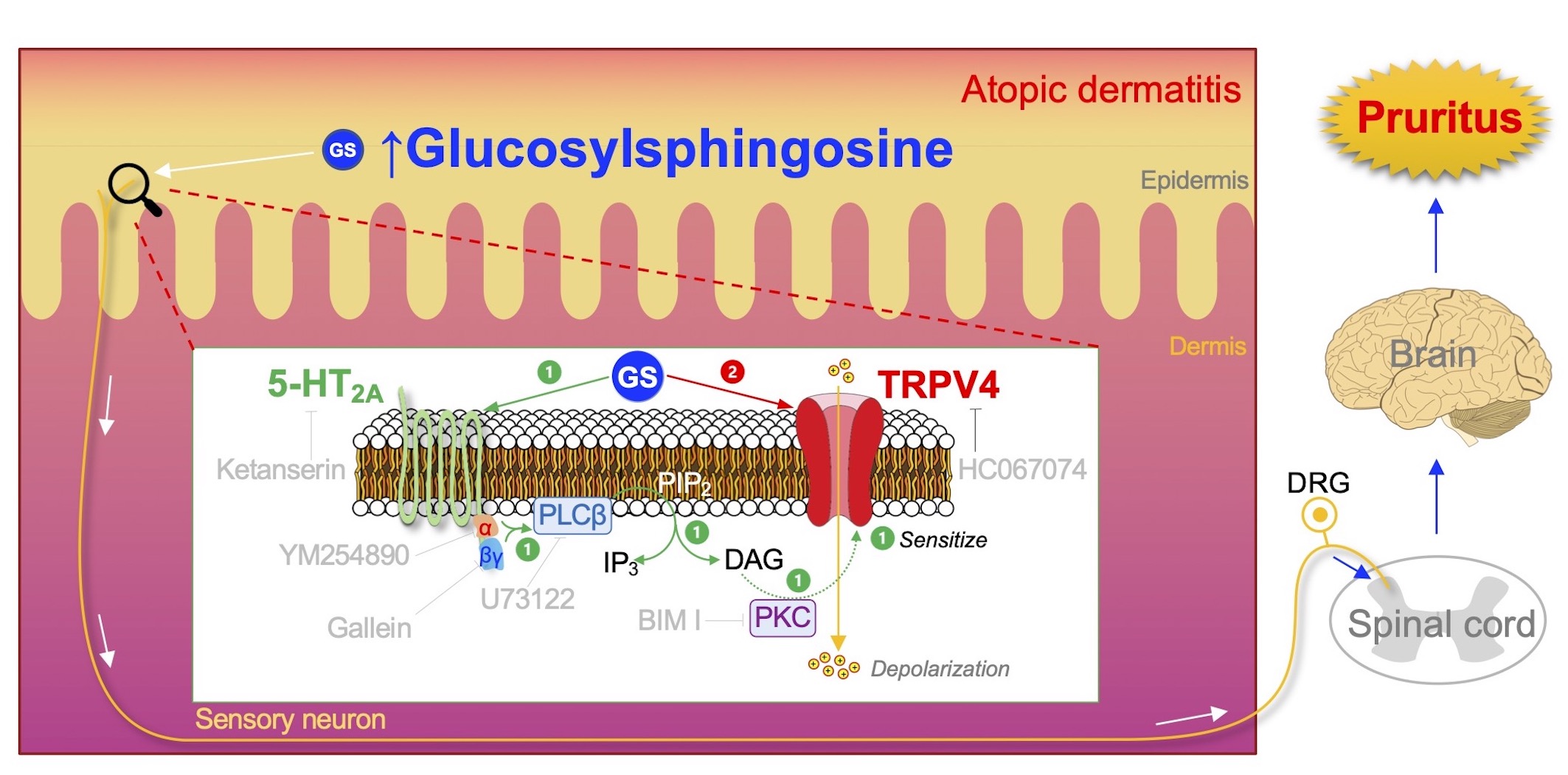
A proposed signalling pathway for Gluocosylsphingosine(GS)-evoked pruritus. The highly accumulated GS in the epidermis of patients with AD will activate the peripheral sensory neuron to induce pruritus in the following way: (1) GS activates 5-HT2A receptor (5-HT2A) with subsequent stimulation of PLC via Gαq/11 and Gβγ complex, which in turn breaks down phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol triphosphate (IP3) and diacylglycerol (DAG). DAG will then activate phosphokinase C (PKC), which leads to sensitization of TRPV4. (2) Activation of the sensitized TRPV4 can be directly facilitated by GS, which will allow a massive influx of cations from the extracellular regions, increasing a chance to induce depolarization and possibly an action potential. This GS-induced electrical signal produced in the peripheral sensory neuron will be further transmitted through the spinal cord and to the brain, where the signal can finally be perceived as pruritus.
Sanjel B, Kim BH, Song MH, Carstens E, Shim WS. Glucosylsphingosine evokes pruritus via activation of 5-HT2A receptor and TRPV4 in sensory neurons.
Br J Pharmacol. 2022 May;179(10):2193-2207.
https://doi.org/10.1111/bph.15733
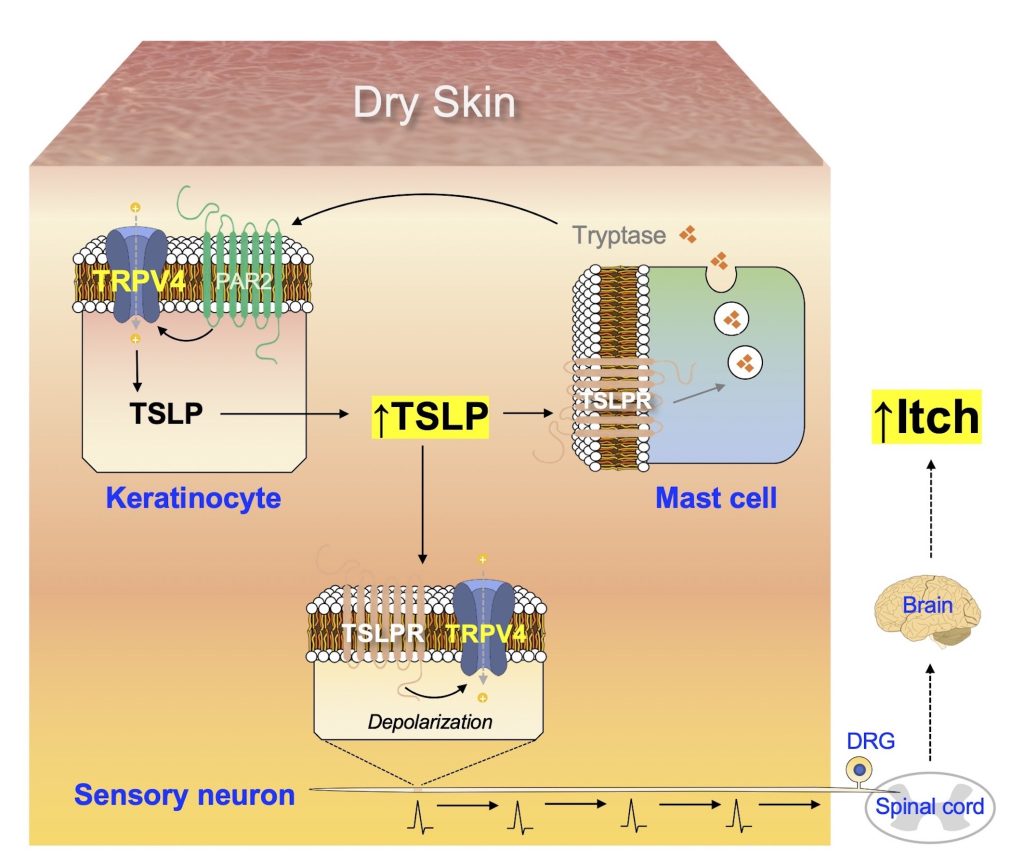
Cutaneous Neuroimmune Interactions of TSLP and TRPV4 Play Pivotal Roles in Dry Skin-Induced Pruritus
Summary of the mechanism underlying dry skin-induced pruritus. The production of TSLP in keratinocytes increases under dry skin conditions, in a TRPV4-dependent manner. Additionally, TSLP acts on TSLPR in mast cells to promote the release of tryptase, which in turn stimulates the keratinocytes via PAR2 and TRPV4 to produce more TSLP, thus generating a positive feedback loop of increased TSLP production. TSLP can also act on TSLPR in sensory neurons to transmit electrical signals towards the spinal cord, which are conveyed to the brain and finally perceived as itch.
Lee WJ, Shim WS. Cutaneous Neuroimmune Interactions of TSLP and TRPV4 Play Pivotal Roles in Dry Skin-Induced Pruritus.
Front Immunol. 2021 Dec 2;12:772941.
Caffeic acid phenethyl ester inhibits pseudo‑allergic reactions via inhibition of MRGPRX2/MrgprB2‑dependent mast cell degranulation
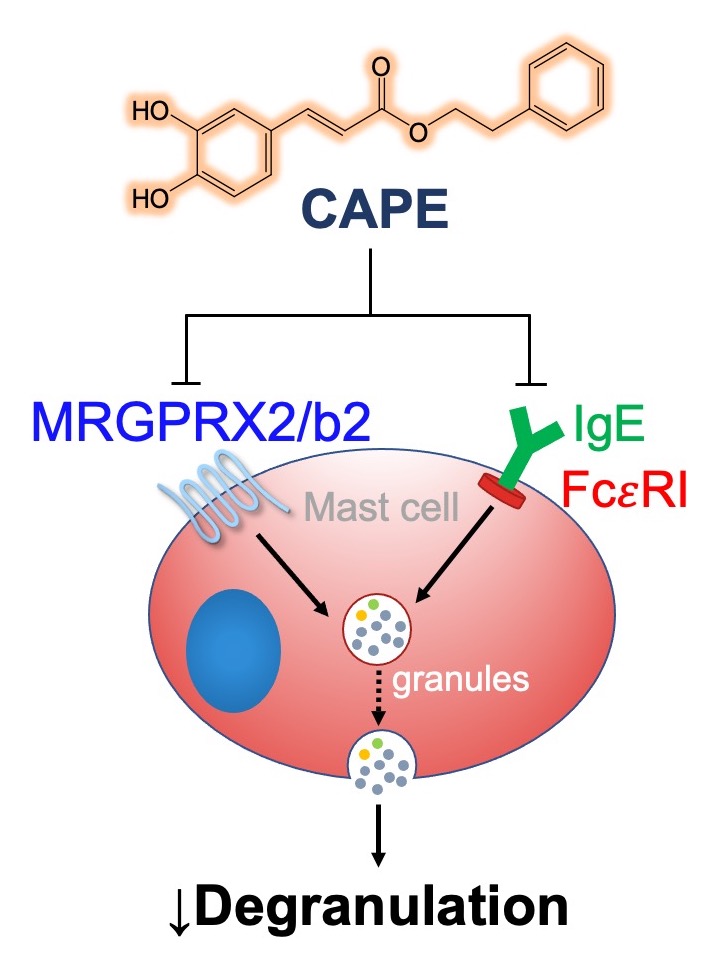
Mast cells play essential role in allergic reactions through the process called mast cell degranulation. Recent studies have found that a basic secretagogue compound 48/80 (C48/80) induces non-IgE-mediated mast cell degranulation via activation of human Mas-related G protein-coupled receptor X2 (MRGPRX2) and mouse MrgprB2. Although previous studies have revealed that caffeic acid (CA) and its derivatives possess anti-allergic effects via IgE-dependent manner, it is largely elusive whether these compounds have impact on MRGPRX2/MrgprB2 to exert inhibitory effects. Therefore, the present study investigated whether CA as well as its derivatives – rosmarinic acid (RA) and caffeic acid phenethyl ester (CAPE) – has the ability to inhibit the activity of MRGPRX2/MrgprB2 to evoke pseudo-allergic effects. As a result, it was found that CAPE inhibits C48/80-induced activation of MRGPRX2/MrgprB2, but neither CA nor RA showed discernible inhibition. Furthermore, the β-hexosaminidase release assay showed that CAPE inhibits mouse peritoneal mast cell degranulation in both IgE-dependent and MrgprB2-dependent manners. Additionally, mouse paw edema induced by C48/80 was dramatically suppressed by co-treatment of CAPE, suggesting that CAPE possesses a protective effect on C48/80-evoked pseudo-allergic reactions. The pretreatment of CAPE also significantly decreased scratching bouts of mice evoked by C48/80, demonstrating that CAPE also has an anti-pruritic effect. Therefore, these data implicate that CAPE can suppress pseudo-allergic reactions evoked by C48/80 via MrgprB2-dependent manner. Finally, molecular docking analysis showed that CAPE is predicted to bind to human MRGPRX2 in the region where C48/80 also binds, implying that CAPE can be a competitive inhibitor of MRGPRX2. In conclusion, it is found that CAPE has the ability to inhibit MRGPRX2/MrgprB2, leading to the prevention of mast cell degranulation and further to the alleviation of mast cell reactions. These results indicate that CAPE as a CA derivative could be developed as a new protective agent that exerts dual inhibition of mast cell degranulation mediated by IgE and MRGPRX2/MrgprB2.
Adhikari N, Shim WS. Caffeic acid phenethyl ester inhibits pseudo-allergic reactions via inhibition of MRGPRX2/MrgprB2-dependent mast cell degranulation.
Arch Pharm Res. 2022 Sep;45(9):644-657
https://doi.org/10.1007/s12272-022-01405-2
