Dual function of MrgprB2 receptor-dependent neural immune axis in chronic pain
Yucui Jiang a1 , Fan Ye d1 , Jian Zhang b1 , Yun Huang b , Yingxin Zong b , Feiyan Chen a , Yan Yang b , Chan Zhu b , Tao Yang c, Guang Yu b , Zongxiang Tang b
aSchool of Chinese Medicine, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing 210023, China
bSchool of Medicine, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing 210023, China
cState Key Laboratory of Organic Electronics and Information Displays & Institute of Advanced Materials (IAM), Nanjing University of Posts & Telecommunications, 9 Wenyuan Road, Nanjing 210023, China
dCollege of Pharmacy, Jishou University, Jishou 416000, China
Received 26 March 2024, Revised 26 November 2024, Accepted 26 February 2025, Available online 28 February 2025.
Abstract
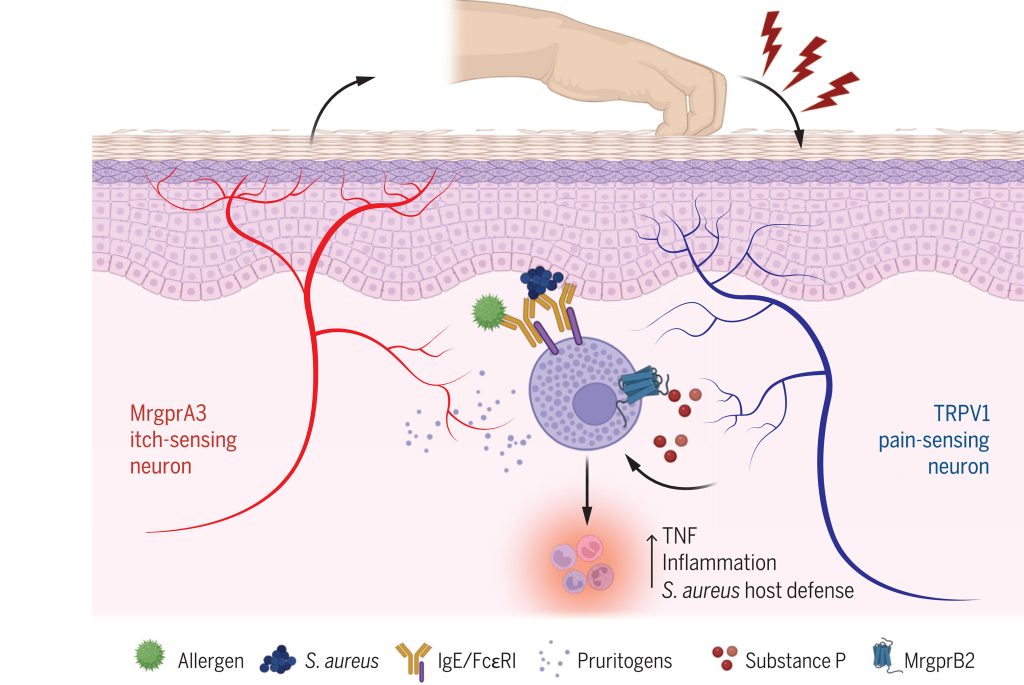
Introduction: Neuro-immune interactions have been recognized to be involved in the development of neuropathic pain induced by chemotherapeutic drugs (CINP). However, its role in pain resolution remains largely unknown, particularly concerning mast cells.
Objectives: To investigate the bidirectional modulation of mast cell Mas-related G protein-coupled receptor B2 (MrgprB2)-mediated neuro-immune interactions in CINP.
Methods: CINP model was established in wild-type mice, Mas-related G protein-coupled receptor D knockout (MrgprD-/-) mice, mast cell-deficient mice, MrgprB2 knockout (MrgprB2-/-) mice, and MrgprB2-Cre tdTomato mice. The role of MrgprB2 receptor in CINP was investigated by calcium imaging, cytokine antibody arrays, mining of single-cell sequencing databases, immunofluorescence, western blotting, co-immunoprecipitation (Co-IP), among other methodologies.
Results: We observed that cisplatin-induced allodynia was significantly inhibited in MrgprB2-/- mice, which was attributed to the blockade of tryptase release and the suppression of upregulation of protease-activated receptor 2 (PAR2) expression in dorsal root ganglion (DRG). Thus, the activation of MrgprB2/Tryptase/PAR2 axis contributed to the development of cisplatin-induced pain. In addition, we also found that there was co-expression of PAR2 and MrgprD in DRG neurons. And activation of PAR2 can negatively regulate the expression of MrgprD, whether in a physiological state or in a chronic pain condition. Consequently, MrgprD expression was down-regulated by the activation of the MrgprB2/Tryptase/PAR2 axis during the later stages of CINP, which was associated with pain relief. Therefore, the activation of MrgprB2/Tryptase/PAR2 axis also contributed to the alleviation of cisplatin-induced pain. This finding was in line with the phenomenon that persistent stimulation by cisplatin did not cause a continuous increase in pain.
Conclusions: Our research elucidated the bidirectional modulation of MrgprB2-dependent neural immune axis in CINP. This study emphasized that MrgprB2 is a critical target for early intervention in CINP, and highlighted the necessity of considering the mechanism differences at different stages in pain management.
Keywords: CINP; MrgprB2 receptor; MrgprD receptor; PAR2 receptor.