Journal club: 26.01.26
Processing of pain and itch information by modality-specific neurons within the anterior cingulate cortex in mice
Hyoung-Gon Ko1,2, Hyunsu Jung#3,4, Seunghyo Han#5, Dong Il Choi#4, Chiwoo Lee#4, Ja Eun Choi4, Jihae Oh4, Chuljung Kwak3, Dae Hee Han3, Jun-Nyeong Kim5, Sanghyun Ye4, Jiah Lee4, Jaehyun Lee4, Kyungmin Lee6, Jae-Hyung Lee7, Min Zhuo8,9 & Bong-Kiun Kaang10,11
1Department of Anatomy and Neurobiology, School of Dentistry, Brain Science and Engineering Institute, Kyungpook National University, 2177 Dalgubeol-daero, Daegu, South Korea. hgko@khu.ac.kr.2Department of Oral Anatomy and Developmental Biology, Kyung Hee University College of Dentistry, Seoul, South Korea. hgko@khu.ac.kr.3Center for Cognition and Sociality, Institute for Basic Science (IBS), Daejeon, 34126, South Korea.4Department of Biological Sciences, College of Natural Sciences, Seoul National University, 1 Gwanangno, Seoul, South Korea.5Department of Anatomy and Neurobiology, School of Dentistry, Brain Science and Engineering Institute, Kyungpook National University, 2177 Dalgubeol-daero, Daegu, South Korea.6Laboratory for Behavioral Neural Circuitry and Physiology, Department of Anatomy, Brain Science and Engineering Institute, School of Medicine, Kyungpook National University, 680 Gukchaebosang-ro, Daegu, South Korea.7Department of Oral Microbiology, College of Dentistry, Kyung Hee University, 26 Kyungheedae-ro, Seoul, South Korea.8Department of Physiology, Faculty of Medicine, University of Toronto, 1 King’s College Circle, Toronto, Ontario, Canada.9International Institute for Brain Research, Qingdao International Academician Park, Qingdao, China.10Center for Cognition and Sociality, Institute for Basic Science (IBS), Daejeon, 34126, South Korea. kaang@ibs.re.kr.11Department of Biological Sciences, College of Natural Sciences, Seoul National University, 1 Gwanangno, Seoul, South Korea. kaang@ibs.re.kr.#Contributed equally.
- PMID: 40038260
- PMCID: PMC11880300
- DOI: 10.1038/s41467-025-57041-z
Abstract
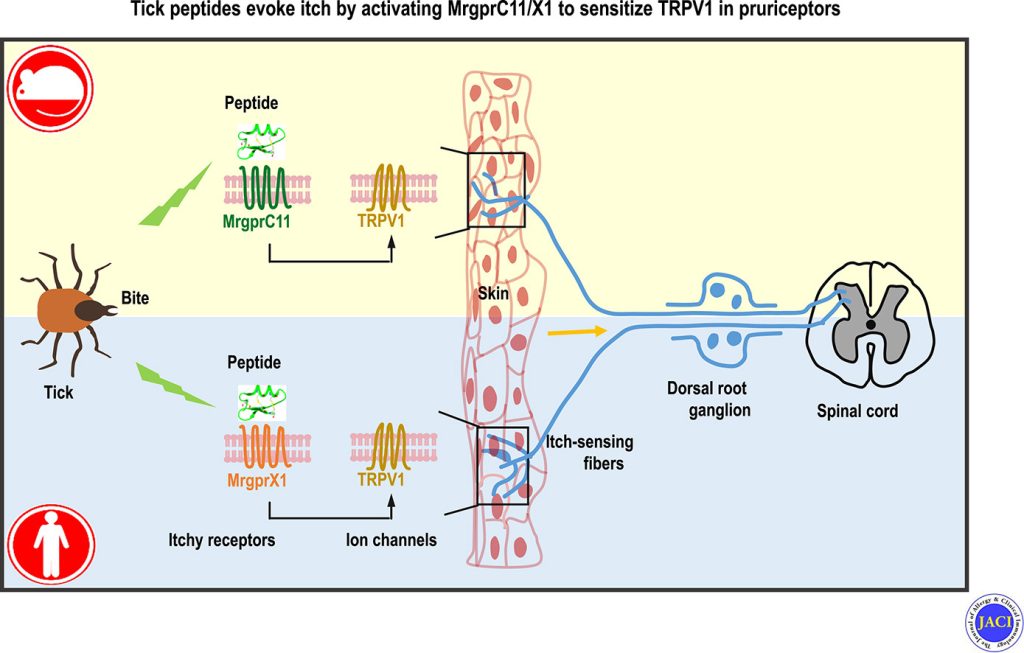
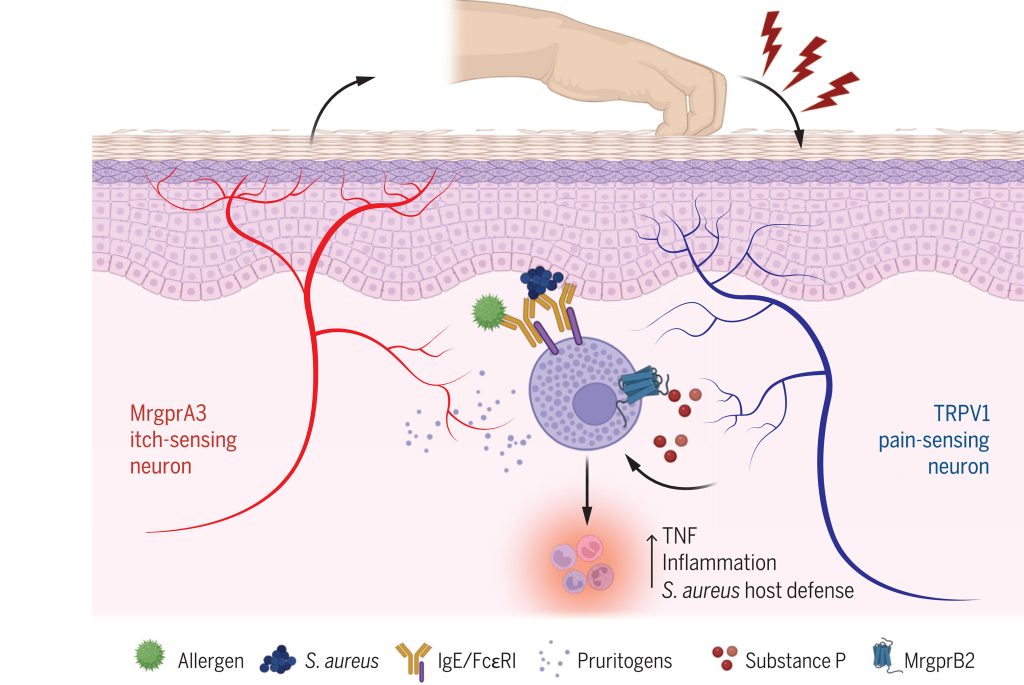
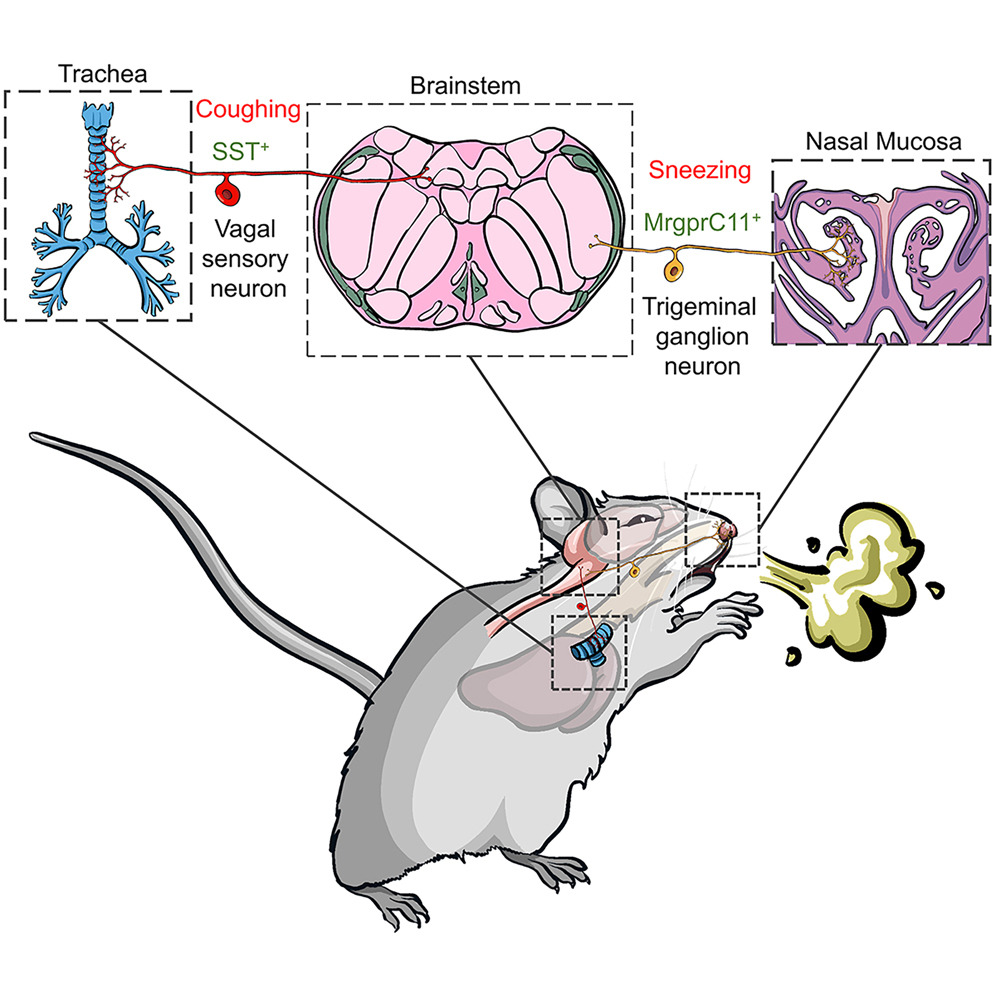
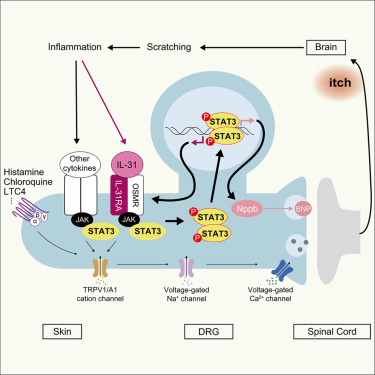
Pain and itch are aversive sensations with distinct qualities, processed in overlapping pathways and brain regions, including the anterior cingulate cortex (ACC), which is critical for their affective dimensions. However, the cellular mechanisms underlying their processing in the ACC remain unclear. Here, we identify modality-specific neuronal populations in layer II/III of the ACC in mice involved in pain and itch processing. Using a synapse labeling tool, we show that pain- and itch-related neurons selectively receive synaptic inputs from mediodorsal thalamic neurons activated by pain and itch stimuli, respectively. Chemogenetic inhibition of these neurons reduced pruriception or nociception without affecting the opposite modality. Conversely, activation of these neurons did not enhance stimulus-specific responses but commonly increased freezing-like behavior. These findings reveal that the processing of itch and pain information in the ACC involves activity-dependent and modality-specific neuronal populations, and that pain and itch are processed by functionally distinct ACC neuronal subsets.
Journal club: 26.01.26 Read More »